Systematically investigate the mechanisms of chemotherapy and immunotherapy resistance in cancer. –Supported by NIH/NCI R01CA255196 (Yang, D) and Shears Family Foundation (Yang, D).
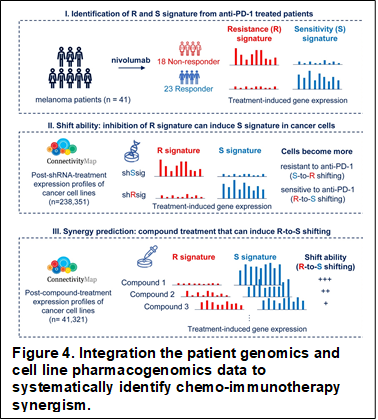
We are among the first researchers to demonstrate that BRCA1 and BRCA2 mutations lead to different genome stabilities and chemotherapy responses in ovarian cancer tumors (JAMA, 2011). Our group have successfully characterized a “BRCAness” phenotype in BRCA1/2 wild type ovarian cancer tumors. Further investigation revealed that loss of RAD50 is a driving mechanism for ovarian cancer BRCAness and cisplatin sensitivity (Gynecologic Oncology, 2016). We later build the first genome-scale data-driven approach for the identification of synthetic viable interactions in cancer genomics, which will help to reveal the mechanism of cancer cell drug resistance (Brief Bioinform, 2017). Most recently, through analyzing anti-PD-1 treatment induced expression changes in patient tumors, we developed an algorithm that can predict whether a chemotherapy treatment shifts anti-PD-1 response. By applying this algorithm on 41,321 compounds and 16,853 shRNA treated cancer cell line expression profiles, we characterized the landscape of chemo-immunotherapy synergism in cancer and experimentally validated doxorubicin and PAK4i to be synergistic with immunotherapy. Further investigation of the treatment induced transcriptomic data revealed that a mitophagy-dependent activation of type I IFN signaling may be a novel mechanism for chemo-immunotherapy synergism. Our study represents the first comprehensive effort to mechanistically characterize chemo-immunotherapy synergism and will facilitate future pre-clinical and clinical studies (Nature Communications, Under Revision, Fig. 4).
- Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, Zhang W. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011 Oct 12;306(14):1557-65. PubMed PMID: 21990299; PubMed Central PMCID: PMC4159096.
- Zhang M, Liu G, Xue F, Edwards R, Sood A, Zhang, W, Yang D#, Copy number deletion of RAD50 as a predictive marker of BRCAness and PARP inhibitor response in BRCA wild type ovarian cancer, Gynecol Oncol. 2016 Apr;141(1):57-64. PubMed PMID: 27016230; PubMed Central PMCID: PMC4967351.
- Gu Y, Wang R, Han Y, Zhou W, Zhao Z, Chen T, Zhang Y, Peng F, Liang H, Qi L, Zhao W, Yang D#, Guo Z#, A landscape of synthetic viable interactions in cancer, Brief Bioinform. 2017 Jan 17. pii: bbw142. doi: 10.1093/bib/bbw142. PMID: 28096076
- Wu Z*, Li S*, Tang X, Wang Y, Guo W, Cao G, Chen K, Zhang M#, Guan M#, Yang D#, Copy Number Amplification of DNA Damage Repair Pathways Potentiates Therapeutic Resistance in Cancer. Theranostics. 2020;10(9):3939-3951; PubMed PMID: 32226530; PubMed Central PMCID: PMC7086350.
- Wang Y, Pattarayan D, Huang H, Zhao Y, Li S, Wang Y, Zhang M, Li S, Yang D#, Systematic investigation of chemo-immunotherapy synergism to shift anti-PD-1 resistance in cancer, Nature Communications, Under Revision.